Dovonex ointment switched from POM to P
In News
Follow this topic
Bookmark
Record learning outcomes
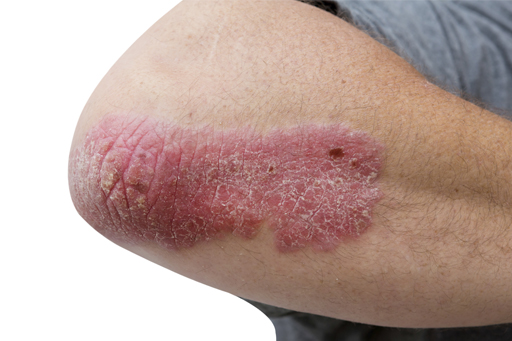
Dovonex Psoriasis Ointment has been reclassified from POM to P, the MHRA announced today (August 17).
Containing the vitamin D analogue calcipotriol 50mcg/g, it is indicated for treating mild to moderate plaque psoriasis that has been previously diagnosed by a doctor in adults aged 18 years and over. The treatment is for application once daily, with a maximum duration of use of 12 weeks and a maximum pack size of 60g.
Dr Sarah Branch, MHRA's deputy director of vigilance and risk management of medicines, said: €Psoriasis is a chronic disease, which can have a major impact on people's quality of life. By making this medicine more widely available, patients will be able to treat flare ups quickly without the need for a prescription.€
The RPS welcomed the news. President Ash Soni commented: €Widening access to medicine is great news for patients. Pharmacists are already able to sell products for the treatment of psoriasis with appropriate advice on management, application and side effects. We welcome the addition of Dovonex Psoriasis Ointment to the range of products available.€
Widening access to medicine is great news for patients
