Latest

Schools now able to purchase AAIs
In Latest
Discover the latest pharmacy news with daily updates and information to keep you in the know.Bookmark
Record learning outcomes
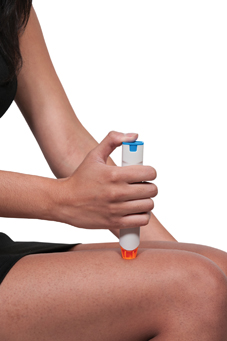 From October 1, 2017, the Human Medicines (Amendment) Regulations 2017 were amended to allow schools to purchase adrenaline auto-injectors (AAI) without a prescription, for emergency use on children who are at risk of anaphylaxis but whose own device is not available or not working.
From October 1, 2017, the Human Medicines (Amendment) Regulations 2017 were amended to allow schools to purchase adrenaline auto-injectors (AAI) without a prescription, for emergency use on children who are at risk of anaphylaxis but whose own device is not available or not working.
This change applies to all primary and secondary schools (including independent schools) in the UK. Schools are not required to hold spare AAI(s) – this is a discretionary, non-statutory change.
The DH has published guidance to help schools that choose to keep emergency AAIs create a policy for using them.
The guidance advises that schools can purchase AAIs from a local pharmacy, without a prescription, provided the general advice relating to these transactions are observed: ie small quantities on an occasional basis and the school does not intend to profit from it.
Pharmacies will need a request signed by the principal or head teacher (ideally on headed paper) stating:
·      the name of the school
·      the purpose for which that product is required
·      the total quantity required.
A template letter which can be used for this purpose is available.
